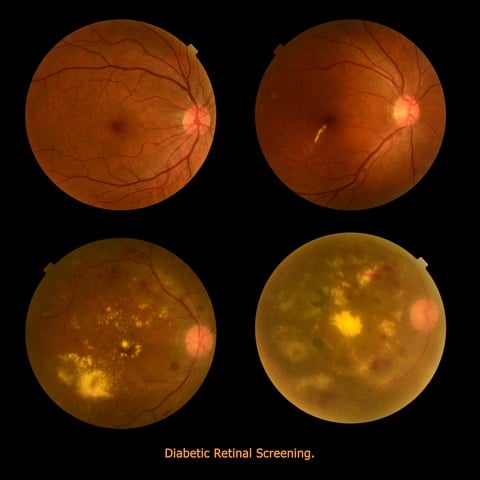
DR could be better treated if detected earlier. However, symptoms are limited and this often prevents a timely and appropriate treatment. Non-proliferative Diabetic Retinopathy (NPDR) is the earlier of the two stages of this pathology. At this stage, fats and other deposits cause micro aneurysms: spots on the walls of blood vessels in the retina. When these outpouch and leak, they cause swelling in the macula and in the nerve fibers. Capillaries may be obtruded and macula stops to work properly due to lack of oxygen, blurring the vision of the patient in the process.
Proliferative Diabetic Retinopathy (PDR) is a later stage of the disease. It occurs when many blood vessels close and then the retina tries to compensate by generating new blood vessels (neovascularization). However, these abnormal vessels do not supply the appropriate amount of blood to the retina. Scar tissues are stimulated by neovascularization, possibly provoking a retinal detachment. Another possible consequence is a damage to the optic nerve resulting in glaucoma.
In terms of imaging, there are several ways to diagnose Diabetic Retinopathy: in the past, the most common method was a fluorescein test during angiography. This modality gives a video clip including the whole process, with fluorescein being visible in the arteries, getting absorbed in tissue and finally going back through the veins. Diabetic Retinopathy screening detects leaks of blood and other elements, giving evidence to the advancement of the disease.
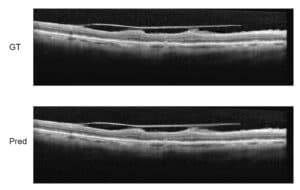
A more modern way to perform Diabetic Retinopathy screening is OCT, which offers the ophthalmologist a very precise angiography image without being invasive for the patient (no injection of substance), which eliminates many side-effects. A third method uses ultrasound to detect Diabetic Retinopathy symptoms: also B-scan ultrasonography enables the screening of blood vessels in the retinal area.
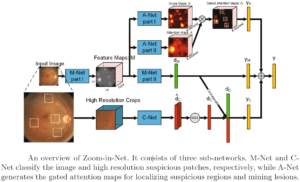
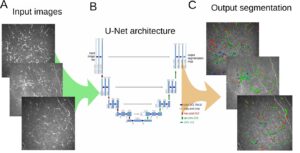
An automated algorithmic approach to detect and quantify Diabetic Retinopathy needs to fulfill several tasks: finding the blood leaks, detecting the growth of new capillaries, which indicate the presence of micro aneurysm. This is done by scanning carefully each pixel in the image, which is a time-consuming process when performed by a human, which justifies the quest for an automated procedure for microaneurysm detection.
This is a tricky problem to solve, since micro aneurysms look like small red dots, very similar to sections of blood vessels typically present in angiography images. Many steps are needed to clear up noise in these images. The first step is preprocessing, including boundary enhancement and more. Following that, we detect those suspected points using other filters like Hough Transform for circles together with other detectors.
This methodology generates many false candidates (false positives) and the following step needs to use machine learning techniques to train a database of micro aneurysms. When enough data is collected, it is possible to use one of the known algorithms for the detection part. In this area, the use of deep learning is very efficient since we can limit the area and give the Convolutional Neural Networks (CNN) enough information to take the decision whether a suspected dot is a micro aneurysm or just a false alarm.

 Ophthalmology
Ophthalmology