Benefits of CMR:
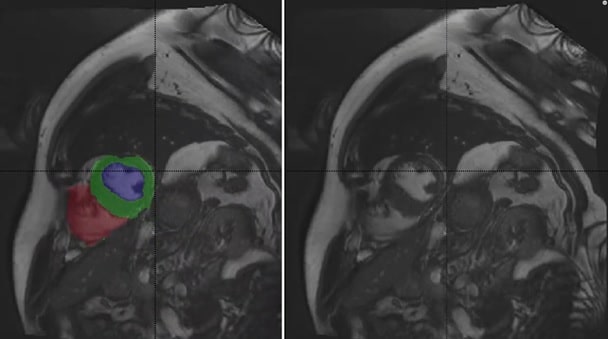
CMR doesn’t require ionizing radiation, as is the case with contrast CT, and its flexibility, high spatial resolution, and 3D capabilities are amenable to assessing myocardial structure and function to identify possible pathologies. The gold standard of cardiac function analysis, CMR gives clinicians insight into ventricular ejection fractions (EF) and stroke volumes (SV), left ventricle mass, and myocardium thickness, however this task requires accurate segmentation of the left ventricular cavity and of the right ventricle for end-diastolic (ED) and end-systolic (ES) phase instances.
Challenges:
Delineating the left ventricle, myocardium and right ventricle from CMR is very common and necessary to establish a diagnosis. However, fully automatic segmentation has not been perfected, meaning that clinicians must continually perform manual segmentation. This is a time-consuming process that is also prone to observer variability.
The success of deep learning in streamlining CMR relies on the automatic performance of two main tasks:
- Segmentation of the left ventricular endocardium and epicardium and the right ventricular endocardium for both ED and ES phase instances
- Classification of the examinations in five classes towards identifying pathologies. The five cases are: normal, heart failure with infarction, dilated cardiomyopathy, hypertrophic cardiomyopathy, abnormal right ventricle.
Towards addressing this challenge, the ACDC provides a fully-annotated dataset for the purpose of CMR assessment, containing data from 150 multi-equipment CMR recordings acquired in routine clinical practice with manual reference measurements and classification from two medical experts.
AI in Cardiac MRI – Methods:
1. Segmentation:
Responding to the first ACDC challenge, over the past two years there has been significant advancement in developing deep learning networks for the automatic segmentation of the LV, LV myocardium, and RV. Generally, the approach applies CNNs based on U-Net and/or ResNet that are trained on the ACDC dataset.
Regression-based methods using novel multiview CNNs utilize long- and short-axis images as input to directly predict left ventricular ejection fraction (LVEF) and left ventricular volumes at ED and ED. Multiscale residual DenseNets have likewise been successfully applied to cardiac segmentation. Other approaches combine deformable models and deep learning method for LV segmentation.
2. Classification:
The successful application of automatic deep learning segmentation from CMR facilitates the classification and diagnosis of cardiac pathologies. One approach trains U-net to automatically analyze CMR images. The current ACDC challenge classification front runner utilized a parameter and memory-efficient FCN architecture based on DenseNets in combination with an up-sampling path and dual loss function to process the input images at multiple scales and viewpoints simultaneously.
Another approach applies a fully automatic neural architecture to accurately and reliably identify the presence, position, transmurality, and size of chronic MI.
Results:
State-of-the-art deep learning segmentation and classification methods for CMR are on par with human expert performance. CNN-based segmentation seems to be even faster and more accurate than manual delineation and also outperforms other state-of-the-art medical imaging technologies while deriving clinically relevant measures necessary for diagnosis.
An additional outcome of automation apart from speed, precision and ease is that these techniques may be optimized to detect and delineate CAD without the need for a gadolinium-based contrast agent.
Conclusion:
Deep learning is being increasingly and successfully applied to facilitate fully-automatic and accurate segmentation of cardiac structures and classifying pathologies in CMR, thereby significantly reducing the postprocessing requirements of cardiac function analysis. This is accelerating patient care workflow, improving accuracy, and reducing clinician workload.