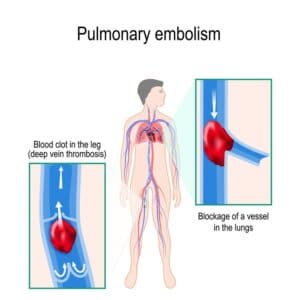
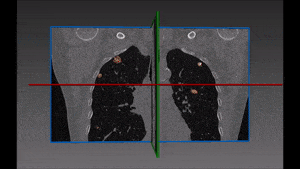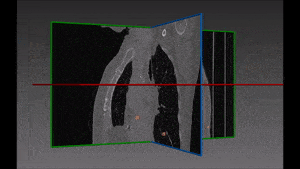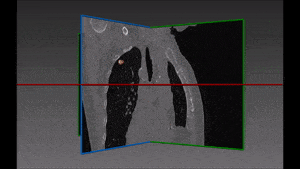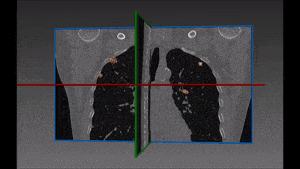
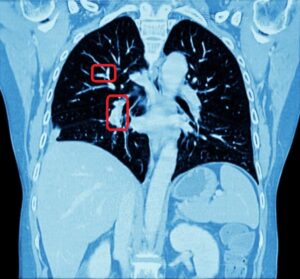
Pulmonary embolism is the obstruction of a blood vessel in the lungs, usually due to a blood clot. It is a very widespread disease, with at least 650,000 cases in the United States every year. It is the third most common cause of death in the US, presenting mortality rates of 25% to 30% when it is not treated in time. Up to 11% of patients die in the first hour following the sudden attack. With proper diagnosis and treatment, 100,000 more patients suffering from pulmonary embolism would survive.

Typically accompanied by generic symptoms (fever, coughing, chest pain), pulmonary arterial obstructions may cause an increase in the right ventricular afterload, potentially leading to life-threatening effects like dilatation, dysfunction and ischemia of the right ventricle. Timely detection via pulmonary CT angiography is key to reduce mortality risks, requiring thus very short scanning times, which are sometimes difficult to achieve in emergency situations and/or with conscious and breathing patients. The end result of challenging conditions might be inadequate image contrast, leading to misleading or slow diagnosis with dramatic consequences.
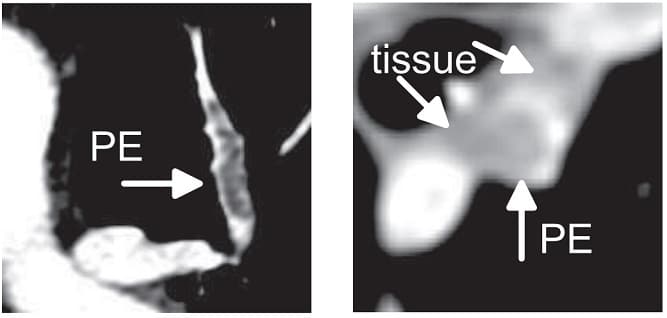
For the above reasons, human detection of pulmonary embolism (the dark spots that correspond to pulmonary emboli in CT angiography images) proves often too slow and can immensely benefit from computer-aided pulmonary embolism detection.
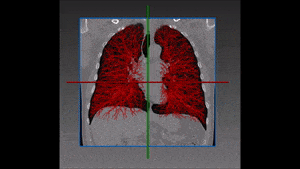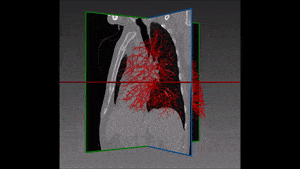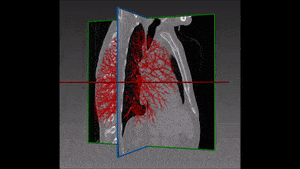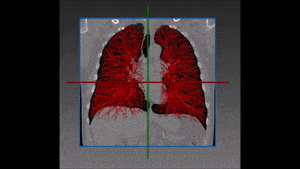
The method which RSIP Vision recommends uses a combination of bidimensional and tridimensional image-processing techniques and subsequently a vessel segmentation procedure which reduces the search area for candidate detection to include the emboli and at the same time exclude a large number of false positives. Particularly, it needs to exclude all lookalikes that present the same intensity of pulmonary emboli: lymphoid tissue, parenchymal diseases and partial-volume voxels on the vessel boundary. Also fully embolized vessels are easily missed by traditional segmentation techniques.
Due to the higher brightness of the larger vessels in the lungs, these are the first to be identified; this is done setting a threshold, the intensity of which is determined in function of the contrast enhancement used. The smaller vessels in the lung are excluded from this process of identification, since their lower intensity in CT image renders them too similar to lymphoid tissue.
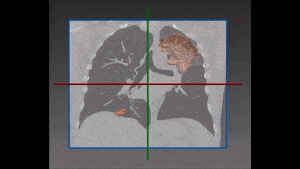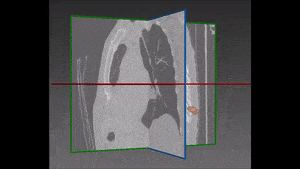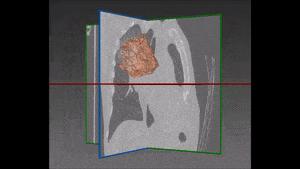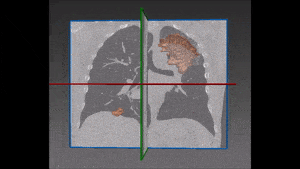
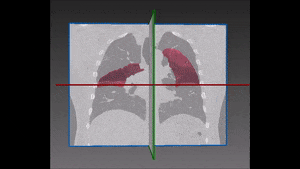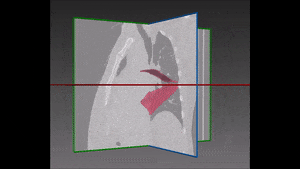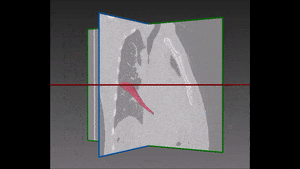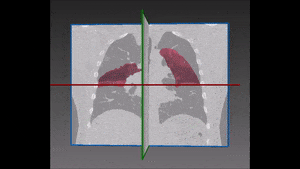
The algorithm looks for local contrast differences within the vessels beyond the given threshold. These contrast variations are then analyzed and obstructions are segmented. Candidate lesions are selected from partial or complete obstruction segmentation. Finally, the measurements coming from the CT such as shape, size and density of the candidate lesions are computed to filter false positives lesions using a decision tree. The remaining candidate lesions found by the algorithm are then identified and treated individually.
The whole procedure must be performed with extreme care, since the patient’s movements, image noise or simply bad quality of the pulmonary CT angiography can have a negative impact on the results, by increasing false positives and excluding pulmonary embolism wherever it should not be found. Also poorly timed contrast injection can preclude the chance from the algorithm to correctly detect the nature of intravascular contrast differences. Statistically, veins, airspace consolidation and lymphoid tissue are the main objects of misinterpretations of contrast differences leading to false positive findings. The always improving image quality of modern computed tomography devices is helping this very effective algorithm procedure to keep false positives to a minimum and by this way it contributes to reduce the dramatic mortality rates of pulmonary embolism, enabling prompter diagnosis and treatment.
Typically accompanied by generic symptoms (fever, coughing, chest pain), pulmonary arterial obstructions may cause an increase in the right ventricular afterload, potentially leading to life-threatening effects like dilatation, dysfunction and ischemia of the right ventricle. Timely detection via pulmonary CT angiography is key to reduce mortality risks, requiring thus very short scanning times, which are sometimes difficult to achieve in emergency situations and/or with conscious and breathing patients. The end result of challenging conditions might be inadequate image contrast, leading to misleading or slow diagnosis with dramatic consequences.
For the above reasons, human detection of pulmonary embolism (the dark spots that correspond to pulmonary emboli in CT angiography images) proves often too slow and can immensely benefit from computer-aided pulmonary embolism detection.
The method which RSIP Vision recommends uses a combination of bidimensional and tridimensional image-processing techniques and subsequently a vessel segmentation procedure which reduces the search area for candidate detection to include the emboli and at the same time exclude a large number of false positives. Particularly, it needs to exclude all lookalikes that present the same intensity of pulmonary emboli: lymphoid tissue, parenchymal diseases and partial-volume voxels on the vessel boundary. Also fully embolized vessels are easily missed by traditional segmentation techniques.
Due to the higher brightness of the larger vessels in the lungs, these are the first to be identified; this is done setting a threshold, the intensity of which is determined in function of the contrast enhancement used. The smaller vessels in the lung are excluded from this process of identification, since their lower intensity in CT image renders them too similar to lymphoid tissue.
The algorithm looks for local contrast differences within the vessels beyond the given threshold. These contrast variations are then analyzed and obstructions are segmented. Candidate lesions are selected from partial or complete obstruction segmentation. Finally, the measurements coming from the CT such as shape, size and density of the candidate lesions are computed to filter false positives lesions using a decision tree. The remaining candidate lesions found by the algorithm are then identified and treated individually.
The whole procedure must be performed with extreme care, since the patient’s movements, image noise or simply bad quality of the pulmonary CT angiography can have a negative impact on the results, by increasing false positives and excluding pulmonary embolism wherever it should not be found. Also poorly timed contrast injection can preclude the chance from the algorithm to correctly detect the nature of intravascular contrast differences. Statistically, veins, airspace consolidation and lymphoid tissue are the main objects of misinterpretations of contrast differences leading to false positive findings. The always improving image quality of modern computed tomography devices is helping this very effective algorithm procedure to keep false positives to a minimum and by this way it contributes to reduce the dramatic mortality rates of pulmonary embolism, enabling prompter diagnosis and treatment.

 Pulmonology
Pulmonology