This article was first published on Computer Vision News of May 2022.
Pallavi Tiwari is an Assistant Professor of Biomedical Engineering and Director of the Brain Image Computing (BrIC) laboratory at Case Western Reserve University. She speaks to us about their innovative work using AI and machine learning in the treatment of brain tumors.
Pallavi, can you tell us about your work?
My lab is developing AI and machine learning-driven approaches for the diagnosis, prediction, and treatment evaluation of neurological disorders, particularly brain tumors. Recently we’ve been looking at post-treatment response assessment. Glioblastoma, one of the most aggressive and highly malignant forms of brain tumor, has a median survival rate of 15 months. Patients know there will be recurrence at some point after treatment. They’re asked to come back for follow-up MRI scans, and if a suspicious lesion is seen, the question is, is it tumor recurrence, or is it just a side effect of the aggressive radiation they’ve received? The only definitive diagnosis comes from surgical resection. Some of these patients, who have already undergone chemoradiation treatment, must undergo another surgery only to realize it was a benign condition and could have been avoided. That affects their quality of life, overall outcomes, and survival.
How do you solve it?
We use AI and machine learning on routinely acquired MRI scans. We don’t look at any fancy imaging. That has allowed us to collaborate with institutions globally to obtain MRI scans. We’re close to 90% accuracy for distinguishing between a benign condition or tumor recurrence. By comparison, for neuroradiologists, the accuracy is close to a coin toss.
How did you end up working with the brain?
My master’s and PhD work at Rutgers was focused on prostate cancer, but I’ve always been fascinated by the brain. My cousin has been suffering from multiple sclerosis for several years. That was another reason for me to delve more into neurological disorders. Towards the end of my PhD, I started talking to a neurosurgeon, who told me how limited the options for brain tumor patients were. That resonated with me, so right after my PhD, I started working in this field.
How good is the community today at tackling brain tumors?
The clinical community still has many questions that need to be addressed. There have been many clinical trials but not much success so far. Multiple trials are coming up to determine if any alternative treatments could work. Compared to other cancers which have moved towards personalized treatments, brain tumor patients get standard chemoradiation. That’s not changed in the last 25 years. Our group has been developing tools and AI and machine learning algorithms to begin to move the needle towards personalized medicine. The aim is to identify patients who might be more suitable for a specific treatment instead of giving them the same aggressive dose of chemoradiation that may be unnecessary.
How is the machine learning community getting involved?
The technical community is recognizing these challenges more and more. A prominent example of that is the BraTS challenge through MICCAI, which has been running for ten years now. Through BraTS and other ongoing brain tumor challenges, more data is being made available, which hugely benefits our community and helps us answer these more challenging questions.
How fast do advancements translate into practical applications in real life?
A big challenge is that groups are working on these problems in a silo. Multiple people must come together both in terms of datasets and algorithms for us to start translating this work into clinical practice. We need a shared resource where we can test each other’s algorithms. To some extent, that’s started to happen now with federated learning and Spyros Bakas’s work at the University of Pennsylvania.
Are brain tumors different for you as a medical imaging expert from other lesions that the human body may have?
One thing that’s challenging about brain tumors is that they’re highly heterogeneous. We presented a paper at MICCAI in 2014 and then published a journal paper in 2016 about a CoLlAGe feature descriptor that captures this intra-tumoral heterogeneity locally. The techniques we’re developing are trying to capture this heterogeneity to predict outcomes in treatment-naive patients and patients after treatment.
Are you able to predict malignancy and the growth patterns of tumors?
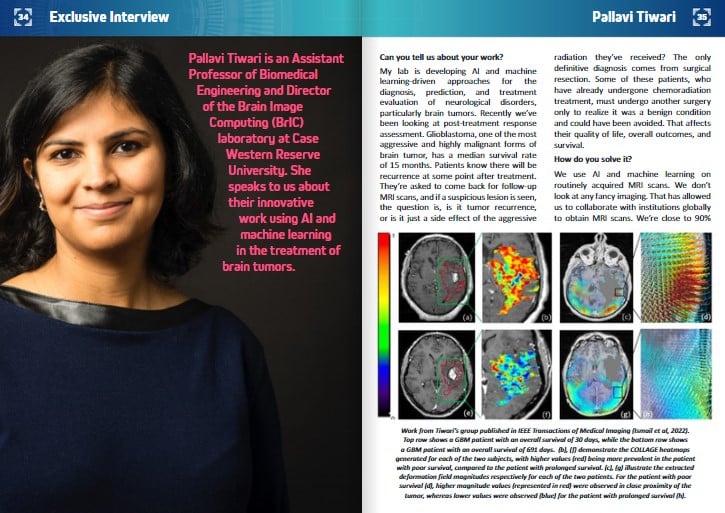
When we think about a brain tumor, it’s like a ball sitting in the patient’s head which is expanding. This ball impacts the rest of the brain in what is known as mass effect. If the tumor grows quickly, this mass effect will impact the rest of the brain. We’ve developed a feature looking at the periphery of the tumor and the region outside it. It captures this mass effect on imaging and uses it to predict how long the patient will live. We compute it by measuring biophysical deformations on routine MR imaging. A paper just came out from our group in IEEE Transactions on Medical Imaging, where we’ve been exploring the impact that the tumor has on the entire brain. Not just thinking about heterogeneity or growth but bringing all these pieces together into a machine learning model and using that model to deduce patient outcomes and their response to specific treatments.
What are the most significant problems brain cancer still has for you and researchers like you?
That’s a great question! There are so many problems I wish we could solve. We’ve already been building solutions for the issue of distinguishing between benign radiation effects and tumor recurrence so that patients can avoid unnecessary aggressive treatment. Another problem is picking the right treatment for the patient. Experimental treatments such as Immunotherapy often don’t work for most brain tumor patients but do for some and have been shown to improve their quality of life and extended their survival. The challenge is to determine which patients will be suitable for immunotherapy or other combination therapies.
Will we see you at MICCAI in Singapore this year?
Most likely!
As one of its early members, can you tell us more about your work with Women in MICCAI?
I care deeply about increasing the visibility of women in MICCAI and medical image analysis. If any women are reading this and are interested in reaching out, I’d be happy to help them or connect them to the right people within the community who would be great resources for them to use.
Have you seen our Women in Science series?
Yes, it’s great you put women on the front page and give them that visibility. That’s also what we’ve tried to do with Women in MICCAI.
Do you have any final thoughts?
I want to give a shout-out to all my students, postdocs, and research associates because they are the driving force behind all of our work. Also, my clinical collaborators and everyone I work with. I’m glad brain tumor is a relatively rare disease given it’s challenges, but this means we don’t have as many patients per institution to work with. Machine learning models need big data. We’ve been fortunate to work with collaborators worldwide who send us scans to evaluate, and we continue to grow this collaboration. They deserve a big shout-out as well for all their help!
Keep reading the Medical Imaging News section of our magazine.